What is the total number of shared pair of electrons in t...
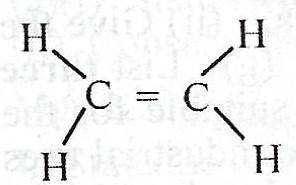
What is the total number of shared pair of electrons in the compound above?
5
8
10
12
Correct answer is D
Each double bond has two pairs of valence electrons shared to form a covalent bonding. Also, every C-H bond, has 2 valence elctrons involved. Drawing the Lewis structure, you see that the above compound has 12 valence electrons shared.
Similar Questions
Which of the following substances is a basic salt? ...
The indicator used in neutralizing CH3COOH and NaOH solution has pH range of...
The gas that is most useful in protecting humans against solar marathon is ...
The oxidation state of oxygen in tetraoxosulphate (VI) acid is ...
Which of the following compounds can exist as geometric isomers? ...
Which of these reagents can confirm the presence of a triple bond? ...