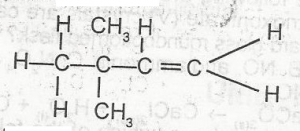
The IUPAC name of the compound above is
2,2-dimethyl but -1-yne
2,2-dmethyl but -1-ene
3,3-dimethyl but -1-ene
3,3-dimethyl but-1-yne
Correct answer is C
No explanation has been provided for this answer.
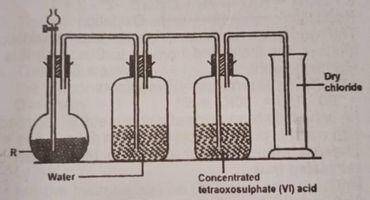
In the diagram above, R is a mixture of?
potassium tetraxochlorate (VII) and concentrated H2SO4
potassium trioxochlorate (V) and concentrated H2SO4
potassium tetratoxomanganate (VII) and concentrated HCI
manganes (IV) oxide and concentrated HCI
Correct answer is D
No explanation has been provided for this answer.
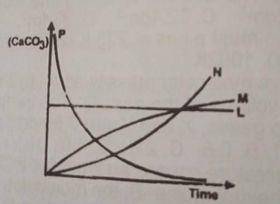
L
M
N
P
Correct answer is D
The concentration of the \(CaCO_3\) decreases as reaction time decreases. therefore option D is correct.
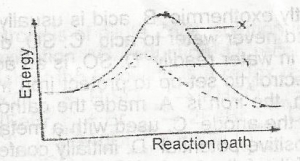
In the diagram above the activation energy is represented by
y - x
x
x - z
y
Correct answer is A
No explanation has been provided for this answer.
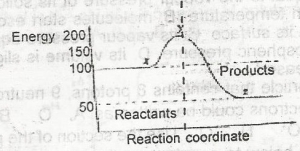
The ∆H for the reaction represented by the energy profile above is
-100 kJ mol--1
+ 100 kJ mol-1
+50 kJ mol-1
-50 kJ mol-1
Correct answer is D
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests