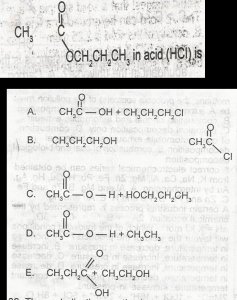
Use the above options to the question. The products formed on hydrolysis of
A
B
C
D
E
Correct answer is C
No explanation has been provided for this answer.
0.2 moles
0.7 moles
1.5 moles
2.0 moles
3.0 moles
Correct answer is D
The molar mass of x = 36g. At 343k, 72g of x dissolved
Molarity of x = \(\frac{72}{36}\) = 2.00moles.
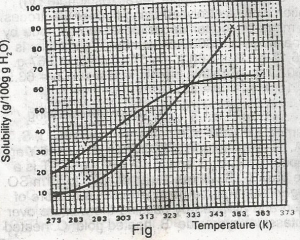
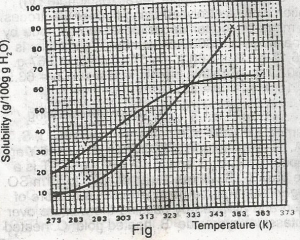
only 10g of X undissolved
only 16 g of Y undissolved
10 g of X and 16 g of Y undissolved
all X and Y dissolved
all X and Y undissolved
Correct answer is B
For salt X, from the graph, 80g of X dissolves by 349K, so by 353K, all 80g of X is dissolved totally in 100g of water. For salt Y, from the graph, 64g of Y dissolves by 353K, so (80-64)g of Y is left undissolved. This is 16g of Y left undissolved at that temperature.
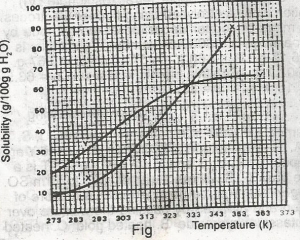
Y is twice as soluble as X
X is twice as soluble as Y
X and Y are soluble to the same extent
X is three times as soluble as Y
Y is three times as soluble as X
Correct answer is A
No explanation has been provided for this answer.
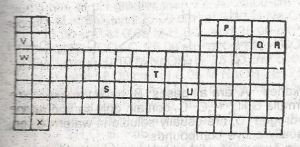
Figure 1 above shows part of the periodic Table which of the elements belongs to the p-block?
S, T and U
V, W and X
S and T only
P, Q and R
V, W, X and S
Correct answer is D
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests