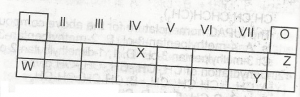
Use the table above to answer this question .The least reactive element is
W
X
Y
Z
Correct answer is D
No explanation has been provided for this answer.
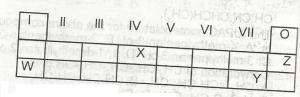
Z
Y
X
W
Correct answer is C
the element likely to participate in covalent rather than ionic bonding is X, covalent bonding is a type of bonding that involves the participants contributing an equal number of electrons into a shared pair.
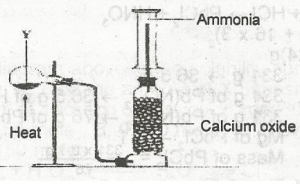
In the diagram above, Y is a mixture of
calcium hydroxide and ammonium chloride
calcuim hydroxide and sodium chloride
sodium chloride and ammonuim trioxonitrate (V)
sodium dioxonitrate (lll) and ammouium chloride
Correct answer is A
No explanation has been provided for this answer.
117.00 g
58.50 g
11.70 g
5.85 g
Correct answer is A
No explanation has been provided for this answer.
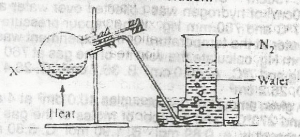
In the experiment above, X could be a solution of
sodium trioxonitrate (V) and ammonium chloride
sodium trioxonitrate (lll) and ammonium chloride
lead (ll) trioxonitrate (V) and copper turnings
potassium trioxonitrate (V) and copper turnings
Correct answer is B
Nitrogen is prepared in the laboratory by heating an equimolar aqueous solution of ammonium chloride and sodium nitrite. The ammonium nitrite formed as a result of a double decomposition reaction, decomposes to form nitrogen gas.
\(NH_4Cl_{(aq)}+NaNO_{2(aq)} → NH_4NO_{2(aq)}+NaCl_{(aq)}\)
\(NH_4NO_{2(aq)} →N_{2(g)} +2H_2O_{(l)}\).
(source: understanding chemistry by G O Ojukuku)
JAMB Subjects
Aptitude Tests