
T
R
J
X
Correct answer is A
No explanation has been provided for this answer.

M and E
G and E
R and L
G and L
Correct answer is D
The alkali metals are found in group one while the noble gases are found in group zero or eight of the periodic table, therefore G and L belong to groups one and eight respectively.
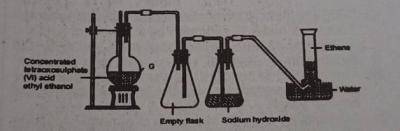
Use the diagram above to answer this question. The reaction taking place in flask G is known as?
hydrolysis
double decomposition
dehydration
pyrolysis
Correct answer is C
The reaction of conc. \(H_2SO_4\) with ethyl ethanol is a dehydration reaction. a process used in producing ethene from alcohol.
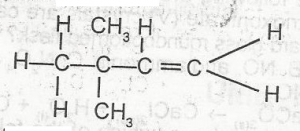
The IUPAC name of the compound above is
2,2-dimethyl but -1-yne
2,2-dmethyl but -1-ene
3,3-dimethyl but -1-ene
3,3-dimethyl but-1-yne
Correct answer is C
No explanation has been provided for this answer.
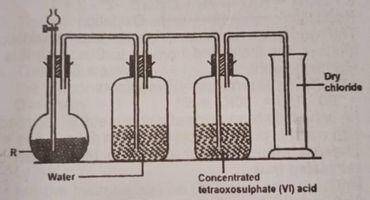
In the diagram above, R is a mixture of?
potassium tetraxochlorate (VII) and concentrated H2SO4
potassium trioxochlorate (V) and concentrated H2SO4
potassium tetratoxomanganate (VII) and concentrated HCI
manganes (IV) oxide and concentrated HCI
Correct answer is D
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests