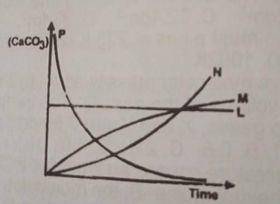
L
M
N
P
Correct answer is D
The concentration of the \(CaCO_3\) decreases as reaction time decreases. therefore option D is correct.
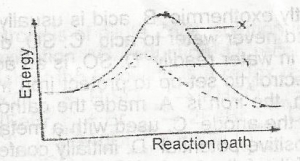
In the diagram above the activation energy is represented by
y - x
x
x - z
y
Correct answer is A
No explanation has been provided for this answer.
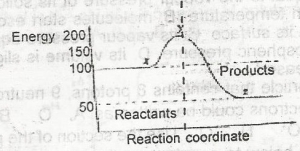
The ∆H for the reaction represented by the energy profile above is
-100 kJ mol--1
+ 100 kJ mol-1
+50 kJ mol-1
-50 kJ mol-1
Correct answer is D
No explanation has been provided for this answer.
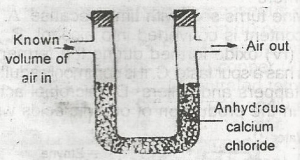
The set-up above would be useful for determining the amount of
oxygen in air
water vapour in air
CO2 in air
arygen in air
Correct answer is B
No explanation has been provided for this answer.
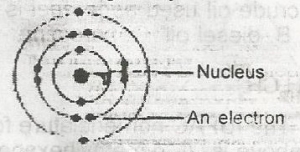
The diagram above represents an atom of
magnesium
helium
chlorine
neon
Correct answer is A
The diagram refers to an atom with electronic configuration 2,8,2 hence the atomic number = 12. From the periodic table, we have the atom to be Mg (Magnesium).
JAMB Subjects
Aptitude Tests