CaSO4
KNO3
NaCl
KCl
Correct answer is B
For many solids that are dissolved in water or any liquid, the solubility increases with a temperature rise. With an increase in kinetic energy, the solvent molecules effectively break apart the solute molecules that are held together by strong intermolecular attractions, and thereby solubility is increased.
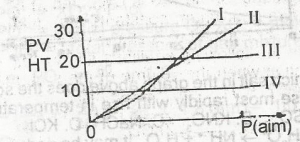
Which of the curves above represents the behaviour of 1 mole of an ideal gas?
l
ll
lll
lV
Correct answer is C
No explanation has been provided for this answer.
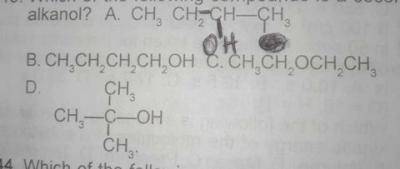
Which of the following compounds is a secondary alkanol?
A
B
C
D
Correct answer is A
Alkanols with two alkyl groups attached to the carbon atom carrying the hydroxyl group is called secondary alkanol
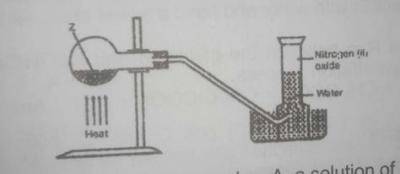
In the experiment above. Z can be
a solution of sodium dioxonitrate (lll) and ammonium chloride
a solution of lead trioxnitrate (V)
a solution of sodium trioxonitrate (V) and ammonium chloride
concentrated tetraoxosulphate (VI) acid and sodium trioxonitrate (V)
Correct answer is C
Nitrogen(I) oxide(laughing gas) can be prepared in the laboratory by the action of heat on a mixture of sodium trioxonitrate (V) and ammonium chloride, this colourless gas is collected over water.
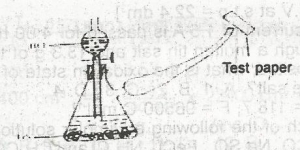
The appropriate test paper to use in the above experiment is moist
litmus paper
potassium heptaoxodichromate (IV) paper
lead (ll) trioxonitrate (V) paper
universal indicator paper
Correct answer is C
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests