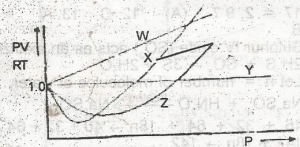
Which of the curves in the above graph illustrates the behaviour of an ideal gas?
W
X
Y
Z
Correct answer is C
No explanation has been provided for this answer.
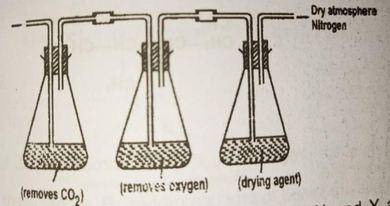
In the above set up substances X and Y are respectively
lime water copper (ll) tetraoxosulphate (lV)
potassium trioxocarbonate and alkaline pyrogallol
potassium hydroxide and alkaline pyrogallol
potassium trioxocarbonate (lV) and concentrated tetraoxosulphate (IV) acid
Correct answer is C
No explanation has been provided for this answer.

It can be deduced from the vapour pressure curves above that
liqiud 1 has the highest boiling point
liquid ll has the highest boiling point
liquid lll has the highest boiling point
liquid lll has the lowest boiling point
Correct answer is C
Trace the iii curve horizontally and you have over 20°C temperature
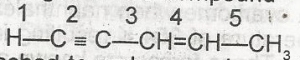
The acidic hydrogen in the compound is the hydrogen attached to carbon number
5
4
3
1
Correct answer is D
No explanation has been provided for this answer.
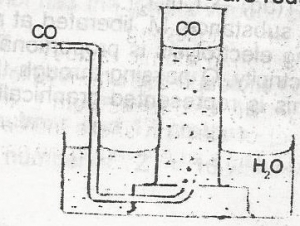
Carbon (ll) oxide may be collected as shown above because it
is heavier than air
is less dense than air
is insoluble in water
burns in oxygen to form carbon (IV) oxide
Correct answer is C
No explanation has been provided for this answer.
JAMB Subjects
Aptitude Tests