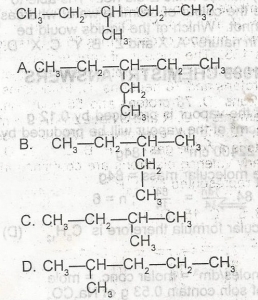
Use the figure above to answer this question. Which of the following is NOT a monomer?
A
B
C
D
Correct answer is A
No explanation has been provided for this answer.
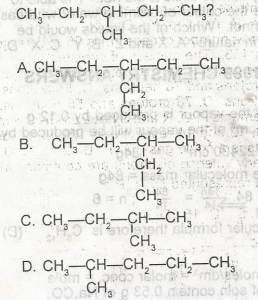
A
B
C
D
Correct answer is D
No explanation has been provided for this answer.
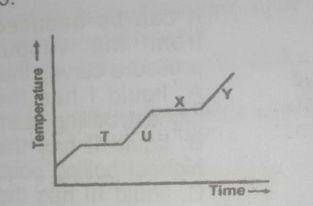
T
U
X
Y
Correct answer is A
point T shows solid-liquid equilibrium. At this point, the substance been heated has begun to melt but has not completely melted forming a solid liquid mixture.
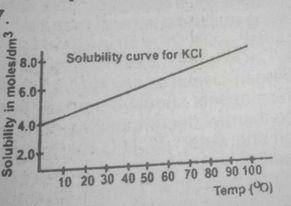
7.45 g
14.90 g
74.50 g
149.00 g
Correct answer is D
Trace the graph to the y-axis to obtain the solubility of the salt at 80°C and at the point where the straight line touches the y-axis, the solubility of the salt at these respective temperatures is 4mol\dm3 and 6mol\dm3 solubility of salt that will crystallize out = 6- 4 = 2mol\dm3 therefore mass of salt deposited = solubility x molar mass of salt 2 x 74.5 = 149.00g
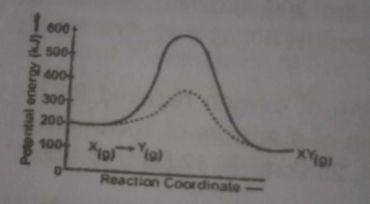
300, 500
500, 300
-300, -500
-500, -300
Correct answer is C
The uncatalysed reverse reaction = 100 - 400 = -300 The calalysed reverse reaction = 100 - 600 = - 500
JAMB Subjects
Aptitude Tests