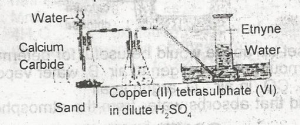
The function of the copper (ll)tetraoxosulphate (IV) in dilute H2SO4 in the figure above is to
dry the gas
absorb phosphine impurity
absorb ethene impurity
from an acetylide with ethyne
Correct answer is B
No explanation has been provided for this answer.
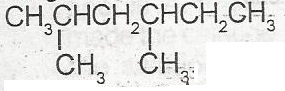
The IUPAC nomenclature for the compound above is
dimethlhexane
3,5 dimethylhexane
1.1 dmethyl, 3 methylpentane
2.4 dimethylhexane
Correct answer is D
No explanation has been provided for this answer.
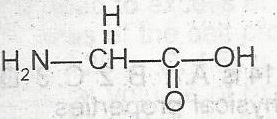
The two functional groups in the above compound are
alcohol and amine
acid and amine
aldehyde and acid
ketone and amine
Correct answer is B
No explanation has been provided for this answer.
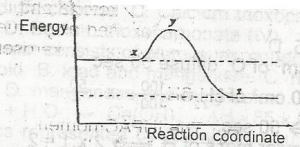
Increase in temperature
Increase in the concentration of a reactant
Addition of a catalyst
Increase in pressure
Correct answer is C
No explanation has been provided for this answer.

Cu
Fe
Ba
Zn
Correct answer is C
Elements with lower electrode potentials are most likely to be reduced and act as a reducing agent. Lower electrode potential indicates a greater tendency to accept electrons.
JAMB Subjects
Aptitude Tests