left and equilibrium constant decreases
left and equilibrium constant increases
right and equilibrium constant increases
right and equilibrium constant decreases
Correct answer is A
This is typical of what happens with any equilibrium where the forward reaction is exothermic. Increasing the temperature decreases the value of the equilibrium constant. Where the forward reaction is endothermic, increasing the temperature increases the value of the equilibrium constant. Endothermic reaction has a Positive Enthalpy change. Exothermic reaction has a negative enthalpy change.
3.30
13.00
10.70
11.30
Correct answer is A
PH+POH=14
p\(^{H}\) = Log [H\(^+\)]
p[H\(^+\) ] = 2.0 X 10\(^{-11}\)moldm\(^{-3}\)
p\(^{H}\) = Log [2.0 X 10\(^{-11}\)]
p\(^{H}\) = 10.70
10.70+ p\(^{OH}\) =14
p\(^{OH}\) = 14 -10.70
= 3.30
law of multiple proportions
law of chemical proportions
law of simple proportions
law of conservation of mass
Correct answer is A
The law of multiple proportion states that when element A and B combine to form more than one chemical compound then the various masses of sample A react with a fixed mass of sample B are in a simple multiple ratio.
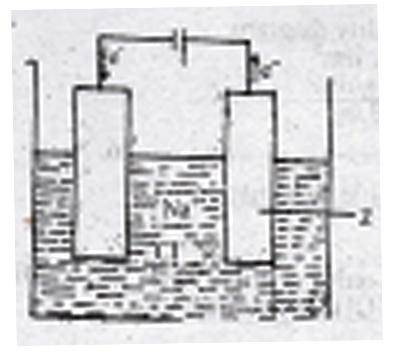
The figure above shows the electrolysis of molten sodium chloride. Z is the
anode where the Cl\(^-\) ions are oxidized
cathode where the Cl\(^-\) ions are reduced
anode where the Na\(^+\) ions are reduced
cathode where the Na\(^+\) ions are oxidized
Correct answer is A
During the electrolysis of brine, the chlorine ions are discharged at the anode and are oxidized. While at the cathode Hydrogen ion is discharged.
Which of the following produces relatively few ions in solution?
NaOH
Ca(OH)\(_2\)
KOH
AI(OH)\(_3\)
Correct answer is D
Aluminium hydroxide is not soluble in water because it is polymeric and it also has high lattice energy and therefore it is hard to break bonds in this compound. So, water cannot dissolve such types of compounds.
Al(OH)\(_3\) is not soluble in water
JAMB Subjects
Aptitude Tests