vibrational motion
vibrational and translational motion
vibrational and random motion
random and translational motion
Correct answer is A
Solids vibrate but generally do not move from place to place.
Which of the following properties would not influence electrovalent bond formation?
Electronegativity
Electron Affinity
Ionization potential
Catalytic ability
Correct answer is D
Every other factor influences the formation of elecrovalent or Ionic bonds except Catalytic ability.
The solubility of \(CO_{2}\) in water can be accounted for by
Van der Waal's forces
Ionic attraction
dipole attraction
covalent bonding
Correct answer is A
In CO2, due to electronegativity difference oxygens have slight negative charge, hence surrounded by polar water molecule forming a cage structure.This leads to its solubility.
chlorine has a larger atomic size
chlorine has a larger atomic mass
chlorine is more electronegative
there is no bonding orbitals within the hydrogen atom
Correct answer is C
The chlorine atom is much more electronegative than the hydrogen atom, which makes this bond polar.
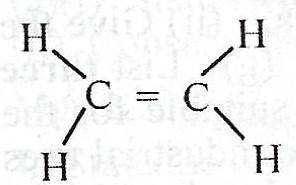
What is the total number of shared pair of electrons in the compound above?
5
8
10
12
Correct answer is D
Each double bond has two pairs of valence electrons shared to form a covalent bonding. Also, every C-H bond, has 2 valence elctrons involved. Drawing the Lewis structure, you see that the above compound has 12 valence electrons shared.
WAEC Subjects
Aptitude Tests