The electron configuration of \(_{26}Fe^{3+}\) is?
[Ar]4s23d6
[AR]4s23d3
[AR]4s13d4
[AR]4s03d5
Correct answer is D
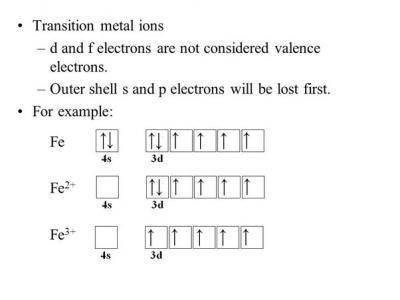
The neutral Iron atom has the electric configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d6.to write the electronic config. of Fe3+ we have to subtract 3 electrons from the outermost shell which is 4s and 3d orbitals. Thus we obtain the electronic configuration for Fe3+ as:
1s2 2s2 2p6 3s2 3p6 4s0 3d5
OR
[AR] 4s0 3d5.
Which of the following processes is used in food preservation in an industry?
Carbon dating
Ieeadiation of gamma rays
Nuclear fission
Nuclear fusion
Correct answer is B
No explanation has been provided for this answer.
Which of the following arrangements is in decreasing order of atomic radius?
Li>Be>C>
Li>B>Be>C
Li>Be>B>C
C>B>Be>Li
Correct answer is C
No explanation has been provided for this answer.
The two elements 11X and 19Y are in the same group because they have the same
valence electrons
ionization energy
number of shell
atomic size
Correct answer is A
No explanation has been provided for this answer.
Hydrogen bonds are formed between molecules containing a hydrogen atom bonded to a
strongly electronegative atom
non-polar species
diatomic element
complex ion
Correct answer is A
No explanation has been provided for this answer.
WAEC Subjects
Aptitude Tests