Which of the following processes is not a surface phenomenon?
Condensation
Evaporation
Photo Emission
Thermionic Emission
Correct answer is A
No explanation has been provided for this answer.
2.0N
3.0N
3.5N
7.0N
Correct answer is A
Upthrust = weight of liquid displaced
= mass of liquid displaced \(\times\) acceleration due to gravity
= \(\frac{200}{1000} \times 10\)
= 2N
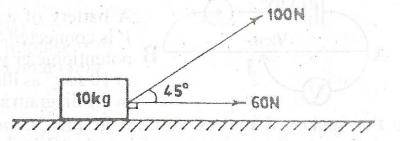
4.0\(ms^{-2}\)
13.4\(ms^{-2}\)
16.0\(ms^{-2}\)
22.6\(ms^{-2}\)
Correct answer is B
Resolving 100N into horizontal and vertical
100 sin45 = 0.7071 * 100 = 70.71
100cos45 = 70.71
Vertical forces = 100 - 70.71 → 29.29
Horizontal force = 70.71 + 60 → 130.71
Resultant of vertical and horizontal forces using pythagoras thoerem
Resultant force (F) = m * a
a = F/m → 133.9/10
a = 13.4
Which of the following is not a measure for reducing balance of payments deficits?
Export drive
Reducing tariffs
Adding to export goods
Increasing local production
Correct answer is B
Tariffs are duties (taxes) imposed on imports. When tariffs are imposed, the prices of imports would increase to the extent of tariff. The increased prices will reduced the demand for imported goods and at the same time induce domestic producers to produce more of import substitutes. Non-essential imports can be drastically reduced by imposing a very high rate of tariff. Therefor, reducing tariffs will further increase balance of payment deficits.
Which of the following statements about matter is not correct?
Molecules of solids move more freely than molecules of liquids and gases
Molecules of solids are more closely packed than those of liquids
Energy is required to break the intermolecular forces of attraction between molecules
Molecules of liquids move about within the liquid and they are in constant motion
Correct answer is A
All other properties of the different states of matter holds except that the molecules of solids don't move freely due to the fact that the particles in the solids are closely packed so it inhibits free motion.
WAEC Subjects
Aptitude Tests