
Physics Questions and Answers
If you want to learn more about the nature and properties of matter and energy or you're simply preparing for a Physics exam, these Physics past questions and answers are ideal for you.

If you want to learn more about the nature and properties of matter and energy or you're simply preparing for a Physics exam, these Physics past questions and answers are ideal for you.
During the nuclear reaction described by
| 235 | W → | 235 | X→ | 231 | Y |
| 92 | 93 | 91 |
α and α
α and β
β and β
β and α
Correct answer is D
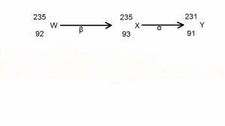
The decay process illustrated above shows that the lost β -particle to decay to the X by increase its atomic number ( from 92 to 93) and leaving the mass no. unaffected. There after, x decayed by -α emission to give Y, by decreasing the mass no. by 2 ( from 93 to 91).
Thus , the emission is β and α particles respectively
X is the anode and very high current is used
X is the anode and Y is the cathode
Y is the cathode and X is the anode
Y is the anode and very high current is used
Correct answer is D
Metals and hydrogen are deposited at the cathode, while non-metals and oxygen are deposited on the anode
∴ metals Y will form the anode which is deposited at the cathode made of x
8.58 x 10-19 J
8.50 x 10-15 J
8.58 x 10-17 J
8.85 x 10-18
Correct answer is A
Work function, w = hfo 6.6 x 10-34 x 1.3 x 1015
= 8.58 x 10-19
saturation
bonding
amplification
dopping
Correct answer is D
No explanation has been provided for this answer.
A radioactive isotope has a decay constant of 10-5s4. Calculate half life
6.93 x 10-5s
6.93 x 105s
6.93 x104s
6.93 x 10-4s
Correct answer is C
T (1)/2 = (0.693)/λ = (0.693)/10-5
= 0.693 x 105
= 6.93 x 104 sec